Abstract
Background: Autologous CD19 CAR T cell therapies have demonstrated favorable clinical responses in relapsed or refractory (R/R) diffuse large B-cell lymphomas (DLBCL), but only a subset of patients (pts) experience durable remissions. We hypothesized that the redundancy of CD28 and CD3V signaling in a CAR design incorporating all 3 CD3V immunoreceptor tyrosine-based activation motifs (ITAMs) might negatively affect T cell differentiation and promote exhaustion. Therefore, we created a new CD19 CAR construct with calibrated CAR activation potential by mutating 2 of the 3 ITAMs, termed 1XX. In systemic ALL mouse models, 19-28z1XX CAR effectively induced tumor eradication at low CAR T cell doses with improved survival compared to conventional 19-28z CAR and with enhanced persistence of functional CAR T cells (Feucht J et al. Nat Med 2019). Herein, we report on adult pts with DLBCL treated with 19(T2)28z-1XX CAR T cells (NCT04464200).
Methods: This is a single-center, phase I, dose-escalation and expansion trial of 19(T2)28z1XX CAR T cells in adult pts with R/R DLBCL conducted at Memorial Sloan Kettering Cancer Center, where CAR T cells were manufactured. Pts received fludarabine and cyclophosphamide lymphodepleting chemotherapy followed by 4 escalating doses of CAR T cells: dose level (DL) 1 (25x106), DL2 (50x106), DL3 (100x106) and DL4 (200x106 CAR T cells). The primary objective of the trial was to evaluate safety and tolerability and determine the recommended phase 2 dose. Key secondary objectives include assessment of overall response and complete response rates. The study has completed enrollment to dose escalation phase.
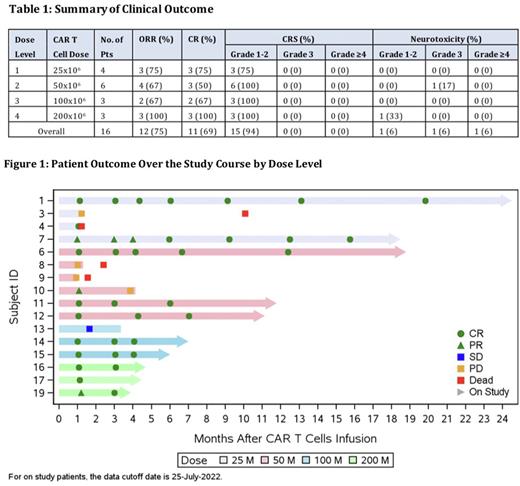
Results: Sixteen pts have been enrolled and treated on the dose escalation phase of the study. Median age was 62 (range, 50-80), and median number of prior treatments was 2 (range, 1-5). Ten pts (63%) had primary refractory disease, 3 pts (19%) had prior autologous HSCT, and 2 pts (13%) had prior autologous CD19 CAR T therapy. 4, 6, 3 and 3 pts were treated at DL1, DL2, DL3 and DL4 CAR T cells, respectively. Overall, 15 pts experienced cytokine release syndrome (CRS) (94%): grade 1 (n=9), grade 2 (n=6). No pts experienced ≥grade 3 CRS. Two pts (13%) experienced neurotoxicity (NTX): 1 pt with grade 1 and 1 pt with grade 3 (Table 1). NTX was transient and reversible in both cases. Eight pts received tocilizumab and 5 pts received corticosteroid.
Overall, 10 of 16 pts (63%) achieved a complete response (CR) and 2 pts (13%) achieved a partial response (PR), with overall response rate (ORR) of 75%. One pt who achieved initial PR spontaneously converted to CR without further treatment after month 6, resulting in best CR rate of 69%. Responses were seen across all dose levels (Table 1). With a median follow-up of 155 days (range, 33-667), no pt in CR has experienced relapse including 7 pts with > 6 months of follow-up (Figure 1).
Peak CAR T cell expansion occurred at a median of 14 days after CAR T cell infusion (range,7-83.3 days). While analysis of CAR T cell persistence is ongoing, CAR T cell detection beyond 350 days has been noted.
Conclusions: Treatment with 19(T2)28z1XX CAR T cells was shown to be safe. No severe CRS was observed and only 1 pt experienced transient grade 3 NTX, while the majority did not experience any. The overall CR rate of 69% is encouraging and appears durable with no relapse observed with a median follow-up of 155 days. CR was observed even at a dose significantly lower (25x106) than those of approved CD19 CAR T cells (100-300x106). These findings corroborates our preclinical data and support that the 1XX signaling domain may lead to enhanced efficacy of CD19 CAR and allow infusion of a lower T cell dose with favorable toxicity profiles. Given that no relevant dose-response relationship was observed, the trial is now moving forward to dose expansion phase with DL1 (25x106 CAR T cells).
Disclosures
Park:Genentech: Research Funding; Novartis: Consultancy; Kite, a Gilead Company: Consultancy; Kura Oncology: Consultancy; AstraZeneca: Consultancy; Juno: Research Funding; Servier: Consultancy, Other: Provision of Services; Intellia: Consultancy; Innate Pharma: Consultancy; Curocell Inc.: Consultancy; Bristol-Myers Squibb: Consultancy; Autolus Therapeutics: Consultancy; Artiva Biotherapeutics, Inc.: Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy; Allogene Therapeutics: Membership on an entity's Board of Directors or advisory committees; Affyimmune Therapeutics, Inc.: Consultancy. Palomba:Magenta: Honoraria; Wolters Kluwer: Patents & Royalties; Nektar: Honoraria; Pluto: Honoraria; Priothera: Honoraria; Rheos: Honoraria; WindMIL: Honoraria; Juno: Patents & Royalties; Kite, a Gilead Company: Consultancy; Novartis: Consultancy; PCYC: Consultancy; Notch: Current equity holder in private company, Honoraria; Seres: Current equity holder in private company, Honoraria, Patents & Royalties, Research Funding; Lygenesis: Honoraria; BeiGene: Consultancy; Ceramedix: Consultancy, Honoraria. Rivière:The Georgia Tech Research Corporation (GTRC): Other: Provision of Services (uncompensated); Mnemo Therapeutics: Current equity holder in private company, Other: Provision of Services (uncompensated), Patents & Royalties; Juno Therapeutics: Patents & Royalties; FloDesign Sonics: Current equity holder in private company, Other: Provision of Services; Fate Therapeutics: Current equity holder in publicly-traded company, Other: Provision of Services, Patents & Royalties; Centre for Commercialization of Cancer Immunotherapy: Other: Provision of Services; Akron Biotechnology, LLC: Other: Provision of Services. Wang:Takeda Pharmaceuticals: Research Funding. Li:Takeda Pharmaceuticals: Current Employment. Zhao:Takeda Pharmaceuticals: Current Employment. Liu:Takeda Pharmaceuticals: Current Employment. Chen:Takeda Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company; Deciphera Pharmaceuticals: Current equity holder in publicly-traded company, Ended employment in the past 24 months. Sellner:Takeda Pharmaceuticals: Current Employment. Sadelain:Takeda Pharmaceuticals: Research Funding; Mnemo Therapeutics: Research Funding; Fate Therapeutics: Research Funding; Atara Biotherapeutics: Consultancy, Patents & Royalties, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal